SGS offers you medical device certification in accordance with Regulation (EU) 2017/745 on Medical Devices (MDR), informally sometimes also called "CE certification”. We provide our service with auditors from Germany and the European region. For your certification process, we support you with a professional and competent team located in Germany. Information about our certification range and the certification process can be found on this page.
Medical devices that are placed on the market in the EU must comply with the European regulations and must be CE marked.
There are currently hundreds of thousands of different types of medical devices. They range from everyday products such as plasters and contact lenses to MRI machines up to state-of-the-art surgical robots. In the EU, the smooth functioning of the internal market and patient safety have been ensured by a common regulatory framework. Medical devices are regulated by the Regulation (EU) 2017/745 on Medical Devices (MDR).
On this page you will find information on basic requirements and legislation for medical devices in the EU, as well as the services that SGS can provide with its Notified Bodies SGS Fimko (Notified Body 0598) and SGS Belgium (Notified Body 1639).
Conformity assessment by a Notified Body
If a medical device belongs to a higher risk class than class I, its conformity with the regulations must be assessed by a Notified Body. After the date of application of the MDR, from May 26, 2021, the assessment must be carried out according to the rules of the MDR.
Depending on the risk class, there are several conformity assessment routes available. We have prepared an infographic on how conformity assessment procedures can be arranged according to MDR Article 52 and what is required in preparing for the conformity assessment.
Our recommendation is to follow the Annex IX route – Conformity Assessment based on a QMS and on the Assessment of Technical Documentation – because MDR prescribes a QMS with minimum requirements for a medical device company.
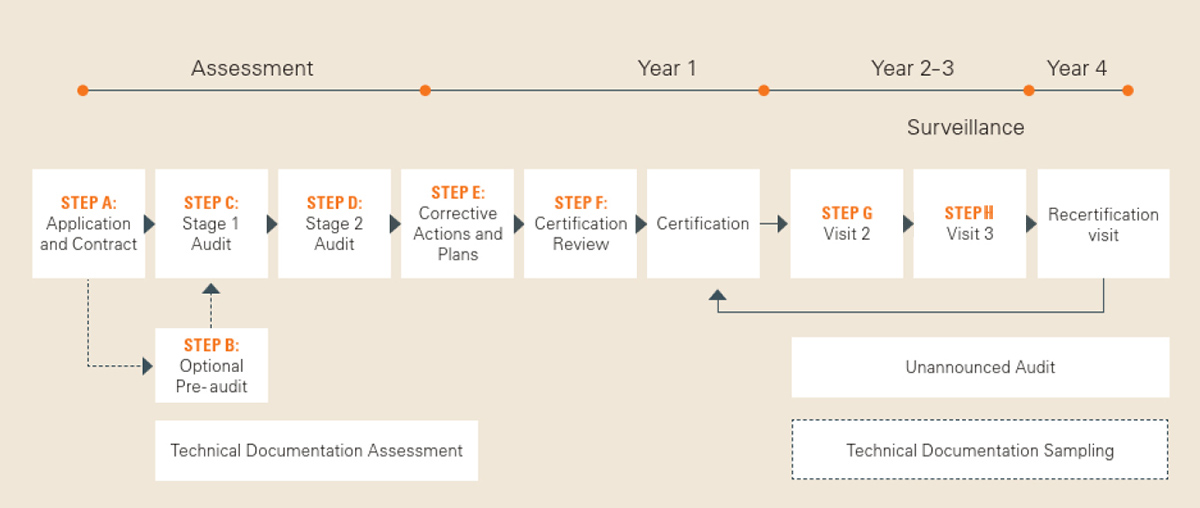
Certification process with SGS
Beginning with an application review, the certification process includes many challenging steps. In the Downloads section you will find our brochure "Your Certification Process Explained", which gives you a good overview of the required steps.
If you are planning to have your medical device certified, please download the forms for your enquiry [Link]. The forms for application for MDR certification with the Notified Body SGS Fimko Oy [Link] are also available here. Please feel free to contact us for further information.
Since medical devices and manufacturers vary greatly, we cannot give you an estimate of the turnaround time or cost for the certification project without an application review. However, you can use our MDR Standard Fees List to get an initial overview of the costs involved.
Additional information
As legal text, the Medical Devices Regulation (EU) 2017/745 (MDR) is not always easy to interpret. For that reason, the Medical Devices Coordination Group is publishing a series of MDCG Guidance Documents.
They include practical guidance on various topics, from EUDAMED to the interpretation of significant changes. Although the MDCG documents are not legally binding, they are highly recommended reading and are applied by Notified Bodies.
Downloads
- Forms for your enquiry about our certification services -> zip download
- Forms for application for MDR certification with the Notified Body SGS Fimko Oy -> zip download
- MDR Client-Technical Documentation Submission – checklist
- MDR Notification of QMS Changes and Regulatory Actions to SGS Fimko Oy -> zip download
- MDR Notification of Product Changes -> zip download
- MD Reporting of EC Vigilance to SGS Fimko Oy -> zip download
- Notification Scope of SGS Fimko Oy
- 10 Steps to CE Mark
Leaflet on 10 Steps for the European CE marking process of Medical Devices according to MDR - Conformity Assessment Routes
An infographic illustrating several available conformity assessment routes according to MDR - MDR Standard Fees List
Costs involved with certification process - Your Certification Process Explained
This important document outlines the audit process for Medical Device Certification according to the Medical Devices Regulation (EU) 2017/745 (MDR)
Contact
Armin Hudetz
t: +49 89 78 74 75-133
E-Mail: This email address is being protected from spambots. You need JavaScript enabled to view it.
Customer Service Team
t: +49 89 78 74 75-222
f: +49 89 12 50 40 64-100
E-Mail: This email address is being protected from spambots. You need JavaScript enabled to view it.