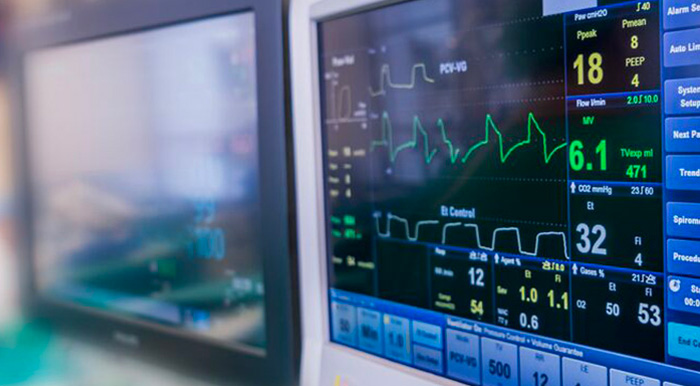
Within the scope of accreditations issued by the DAkkS (ILAC ISO 17025) and in the IECEE CB Scheme, SGS Germany GmbH offers the full range of product safety and EMC certifications for patient monitors of all kinds.
Patient monitors are generally used to display and monitor the physiological parameters of a patient. The top models on the market offer extensive options to display the vital data of a patient with the help of various sensors (applied parts) and thus to draw conclusions on the patient’s state of health. Frequently monitored values are the heart rhythm (ECG), blood pressure (IBP or NIBP), oxygen saturation (SpO2), body temperature, and the carbon dioxide content in the exhaled air (etCO2).
For each of these parameters, there are specific normative requirements for functionality and accuracy. These requirements are specifically outlined in the particular standards (IEC/ISO 60601/80601-2-xx). Verification is conducted using specially developed test and simulation equipment.
In addition to these function-specific requirements, there are other conditions that must be fulfilled depending on the intended use of the patient monitor.
In intensive care, i.e. when monitoring a patient who is acutely ill or undergoing an operation, the monitor must be equipped with an alarm system according to IEC 60601-1-8. If the physiological values of the patient register outside certain, usually adjustable, limits, the healthcare staff is alerted by visual and audible alarms and can initiate appropriate measures. But there are also technical alarms, such as for a low battery charge in internally supplied devices, to indicate a malfunction.
For patient monitors that are also to be used outside medically used rooms, for example by lay operators or emergency paramedics, IEC 60601-1-11 (home healthcare environment) or IEC 60601-1-12 (EMS – emergency medical services environment) must also be applied.
The two standards account for the less controlled environmental conditions in these areas and therefore require the patient monitor to be more robust against external influences.
We carry out the complete range of these environmental tests (pressure and climate storages, IP tests, vibration and shock tests) at our new location in Geretsried-Gelting near Munich.
For the testing of patient monitors, SGS Germany GmbH holds accreditations according to the following standards, among others:
|
Number |
Title English |
|---|---|
| DIN EN 60601-1:2013 IEC 60601-1:2005/AMD1:2020 IEC 60601-1:2005/AMD2:2020 |
Medical electrical equipment – Part 1: General requirements for basic safety and essential performance |
| DIN EN 60601-1-2:2016 IEC 60601-1-2:2014 IEC 60601-1-2:2014/AMD1:2020 |
Medical electrical equipment – Part 1-2: General requirements for basic safety and essential performance – Collateral standard: Electromagnetic disturbances – Requirements and tests |
| DIN EN 60601-1-6:2016-02 IEC 60601-1-6:2010/AMD1:2013 IEC 60601-1-6:2010/AMD2:2020 |
Medical electrical equipment – Part 1-6: General requirements for basic safety and essential performance - Collateral standard: Usability |
| DIN EN 60601-1-8: 2014-04 IEC 60601-1-8:2006/AMD1:2012 IEC 60601-1-8:2006/AMD2:2020 |
Medical electrical equipment – Part 1-8: General requirements for safety – Collateral standard: General requirements, tests and guidance for alarm systems in medical electrical equipment and medical electrical systems |
| DIN EN 60601-1-11: 2016-04 IEC 60601-1-11:2015 IEC 60601-1-11:2015 / AMD1:2020 |
Medical electrical equipment – Part 1-11: General requirements for basic safety and essential performance – Collateral Standard: Requirements for medical electrical equipment and medical electrical systems used in the home healthcare environment |
| DIN EN 60601-1-12:2016 IEC 60601-1-12:2014 IEC 60601-1-12:2014 / AMD1:2020 |
Medical electrical equipment – Part 1-12: General requirements for basic safety and essential performance – Collateral Standard: Requirements for medical electrical equipment and medical electrical systems intended for use in the emergency medical services environment |
| DIN EN 60601-2-4:2012 IEC 60601-2-4:2010 IEC 60601-2-4:2010/AMD1:2018 |
Medical electrical equipment – Part 2-4: Particular requirements for the basic safety and essential performance of cardiac defibrillators |
| DIN EN 60601-2-25:2016 IEC 60601-2-25:2011 |
Medical electrical equipment – Part 2-25: Particular requirements for the basic safety and essential performance of electrocardiographs |
| DIN EN 60601-2-27:2015 IEC 60601-2-25:2011 |
Medical electrical equipment – Part 2-27: Particular requirements for the basic safety and essential performance of electrocardiographic monitoring equipment |
| DIN EN 80601-2-30:2020 DIN EN 80601-2-30:2016 IEC 80601-2-30:2009 / AMD1:2013 IEC 80601-2-30:2018 |
Medical electrical equipment – Part 2-30: Particular requirements for the basic safety and essential performance of automated non-invasive sphygmomanometers |
| DIN EN 60601-2-34:2015 IEC 60601-2-34:2011 |
Medical electrical equipment – Part 2-34: Particular requirements for the basic safety and essential performance of invasive blood pressure monitoring equipment |
| DIN EN IEC 80601-2-49:2020 IEC 80601-2-49:2018 DIN EN 60601-2-49:2016 IEC 60601-2-49:2011 |
Medical electrical equipment – Part 2-49: Particular requirements for the basic safety and essential performance of multifunction patient monitoring equipment |
| DIN EN ISO 80601-2-55:2019 ISO 80601-2-55:2018 |
Medical electrical equipment – Part 2-55: Particular requirements for the basic safety and essential performance of respiratory gas monitors |
| DIN EN ISO 80601-2-56:2020 ISO80601-2-56:2017/AMD1:2018 |
Medical electrical equipment – Part 2-56: Particular requirements for basic safety and essential performance of clinical thermometers for body temperature measurement |
| DIN EN ISO 80601-2-61:2019 ISO 80601-2-61:2017 |
Medical electrical equipment – Part 2-61: Particular requirements for basic safety and essential performance of puls oximeter equipment |
Contact
Armin Hudetz
t: +49 89 78 74 75-133
f: +49 89 12 50 40 64-133
E-Mail: This email address is being protected from spambots. You need JavaScript enabled to view it.
Customer Service Team
t: +49 89 78 74 75-222
f: +49 89 12 50 40 64-100
E-Mail: This email address is being protected from spambots. You need JavaScript enabled to view it.