The European Commission has published two new documents, which detail the rules on EU market access for manufacturers of devices without medical intended purpose listed in Annex XVI of MDR.
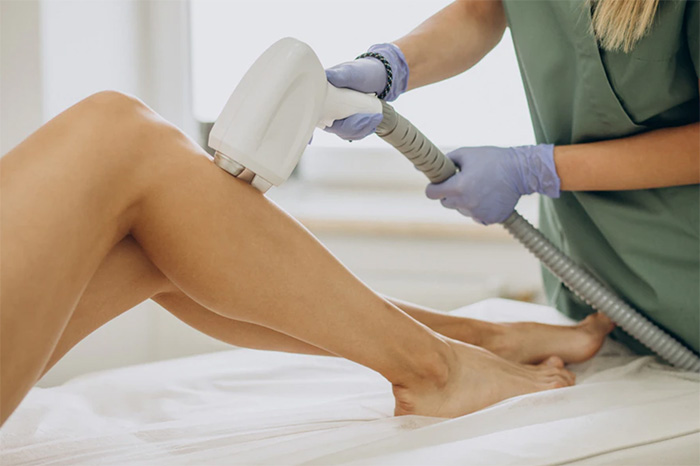
Common specifications (CS) for skin treatment devices using infrared, visible light, and ultraviolet have been published in Annex VI of Regulation (EU) 2022/2346.
Also, other products, e.g., devices intended for liposuction, lipolysis, or lipoplasty, are now covered by the common specifications.
Regulation (EU) 2022/2347 describes how MDR rules have to be applied and what conformity assessment routes can be utilized by manufacturers bringing medical devices without intended medical purpose onto the EU market.
Our Notified Body, SGS Fimko Oy, has Annex XVI in its scope. We offer MDR certification for the products falling in scope of the newly issued common specifications, including laser and IPL skin treatment products.
You are welcome to contact us by This email address is being protected from spambots. You need JavaScript enabled to view it. for further explanations concerning the MDR certification process we will contact you immediately.
Contact
Armin Hudetz
t: +49 89 787475-133
f: +49 89 12504064-133
E-Mail: This email address is being protected from spambots. You need JavaScript enabled to view it.
Customer Service Team
t: +49 89 787475-222
f: +49 89 12504064-100
E-Mail: This email address is being protected from spambots. You need JavaScript enabled to view it.
