The list of harmonized standards under Medical Device Regulation (EU) 2017/745 was amended with the latest normative documents concerning respiratory devices and sterile medical products. The deadline for adoption of the standards by the manufacturers is set for May 27, 2024. More details can be found on the European Commission eNorm Platform (europa.eu)
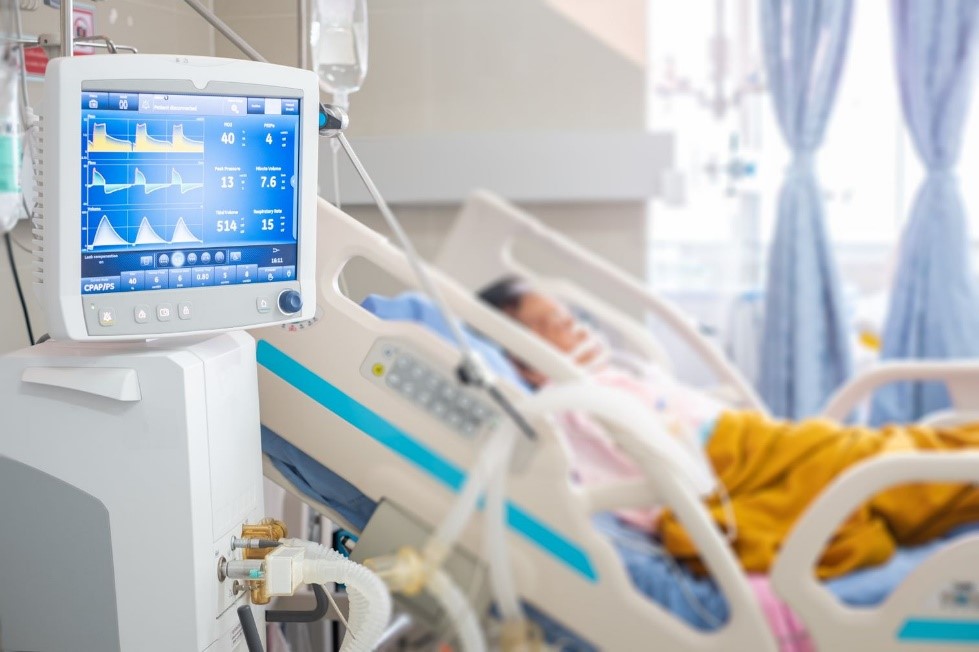
SGS offers product safety testing and MDR certification for medical devices, including respiratory devices. Visit our webpage to find out more about the services we provide from Munich in Germany: Medical Device Services
We are able to offer the certification service in a timely manner, without long waiting times. You are welcome to contact us via This email address is being protected from spambots. You need JavaScript enabled to view it.. We will explain our MDR certification process at SGS to you and answer your questions.
Contact
Armin Hudetz
t: +49 89 78 74 75-133
f: +49 89 12 50 40 64-133
E-Mail: This email address is being protected from spambots. You need JavaScript enabled to view it.
Customer Service Team
t: +49 89 78 74 75-222
f: +49 89 12 50 40 64-100
E-Mail: This email address is being protected from spambots. You need JavaScript enabled to view it.
Related links
Publication of updated list of harmonized standards for MDR
Information about our Medical Device Services
Information about our MDR certification service
Notification Scope of SGS Fimko Oy
SGS Fimko Ltd NB 0598 Standard fees list for MDR (EU) 2017/745 EC Certification
